STRUCTURE AND POWER OF ATOMS
Everything visible in the universe, all matter, is made up of an uncountable number of atoms coming together in different ways. But how do atoms come together? Let's take a look at what happens in the world of atoms.
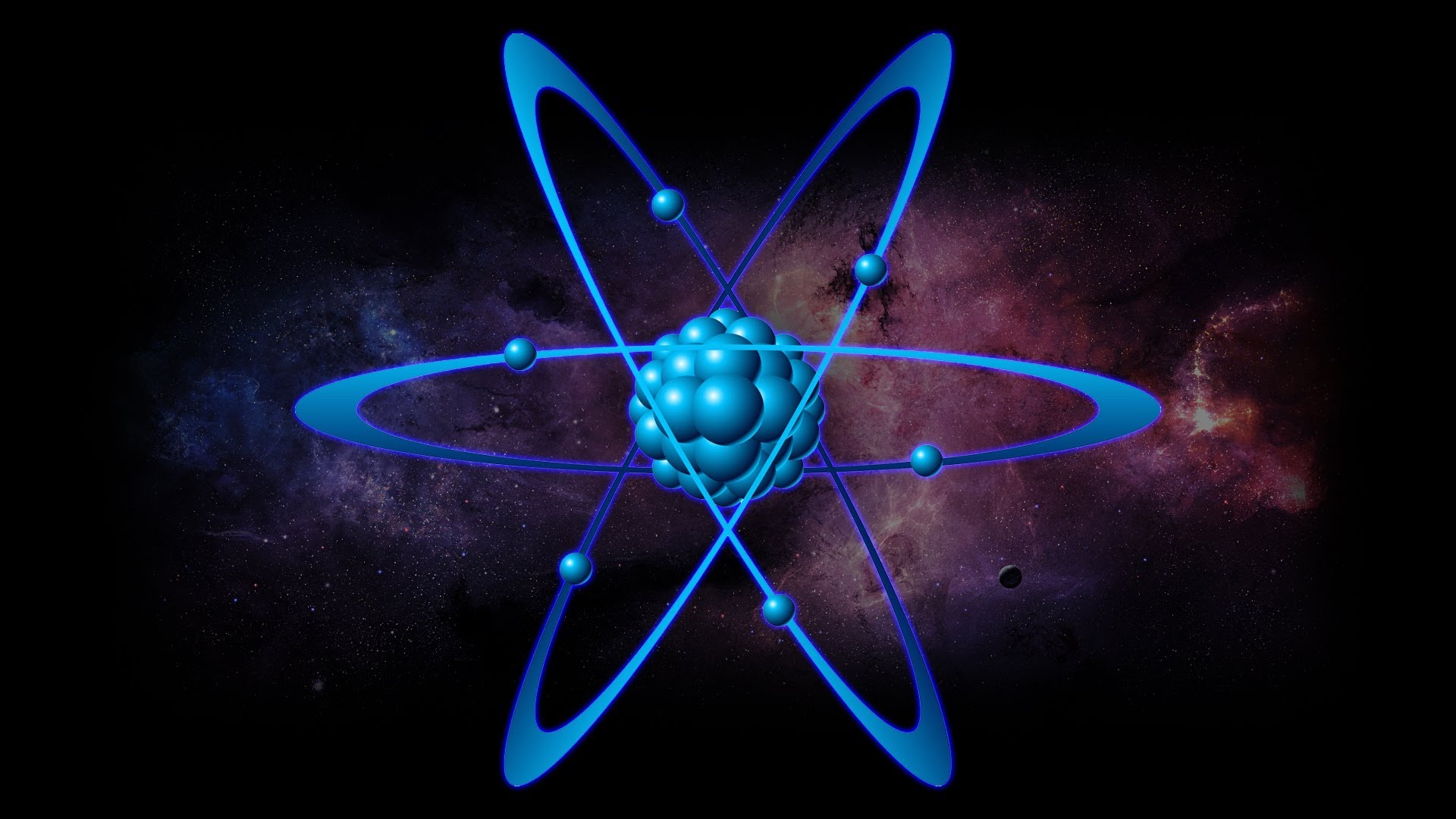
What brings atoms together is electric charge. Yes, atoms can come together through electric charge. At the center of every atom is a nucleus. This nucleus is made up of particles called protons and neutrons. Protons are positively charged particles and neutrons are uncharged. In other words, they are neutrally charged.
Around this small structure called the nucleus are even smaller negatively charged particles called electrons. Normally, atoms have an equal number of protons and electrons. Because of their opposite charges, protons and electrons attract each other. This is what holds the atom together. Because of this balance, atoms are electrically neutral.
But there is one small problem: these electrons are unstable things:
They can be attracted not only to the nuclei of their own atoms, but sometimes to the nuclei of other atoms as well. So they are not very loyal to the structure to which they are attached.
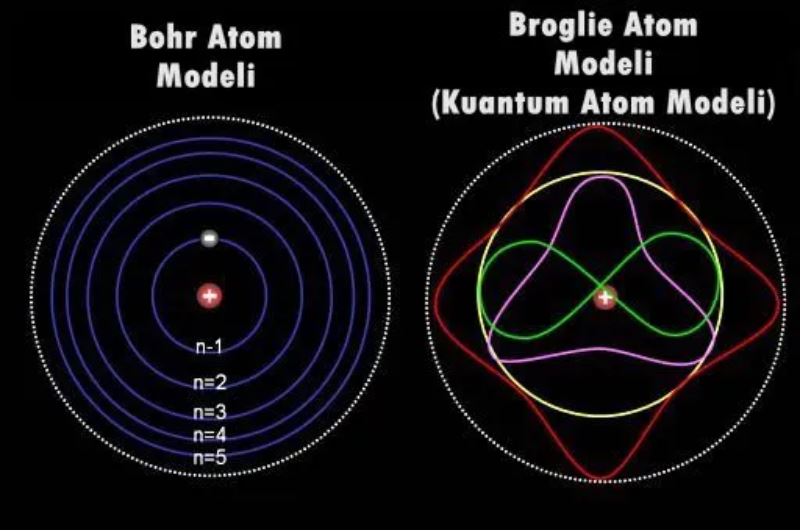
The shell at the lowest energy level contains at most two electrons, and the next level contains at most eight electrons. To achieve maximum stability, electrons settle at the lowest energy level where there are vacancies.
An important factor in chemical bonding is the number of vacancies in the outermost shell of atoms, called the valence shell. With the right combination of vacancies, electrons can jump from atom to atom, two atoms can share the same electron, or many atoms can share an electron cloud. Atoms are more stable when the valence shell is full. So electrons try to move in such a way that they form full valence shells. So in a sense, the electrons we accuse of being unfaithful are actually moving for more stable molecular structures.
We call the structures formed by multiple atoms bonding with each other molecules.
Molecules can be composed of identical atoms (atoms of the same element) or atoms of different elements. Molecules composed of at least two different elements are called “compounds”.
The materials we use in daily life are made up of many molecules.
The structure of each molecule and the way they come together determines how the material feels and behaves.
In general, molecules can come together in three ways: gas, liquid and solid. In gases, molecules move freely. In liquids, molecules come together loosely and slide over each other like marbles in a bowl. In solids, molecules cannot move freely because they are organized into more rigid structures.
The different combinations and arrangements of atoms create an incredible variety of qualities and behaviors.
Even in identical atoms, structural changes can make a big difference. Take diamond and graphite, for example. Both are made of carbon atoms, but graphite is a worthless material used to make pencils.
In diamond, strong covalent bonds bring atoms together in a solid lattice structure. The result is one of the world's hardest and toughest materials. In graphite, carbon atoms are arranged in a layered structure and the bonds between the layers are very weak. So weak that we can break those bonds by rubbing a pencil on paper. The ability to assemble and arrange atoms into different structures opens up unimaginable possibilities. Scientists and engineers have developed thousands of new materials in this way, and the possible combinations are endless.
The chemical reactions that bring atoms together also have some benefits. Fire, for example, is the result of a chemical reaction between chemical compounds in wood (or other fuel) and oxygen in the atmosphere, triggered by intense heat. Burning wood produces gaseous compounds of hydrogen, carbon and oxygen, as well as charred residues. As the gases heat up, the compounds break down and the atoms recombine with oxygen in the air to produce water, carbon dioxide, carbon monoxide and nitrogen. This process releases a huge amount of energy in the form of heat and light.
Manipulating atoms has always been at the center of human technology. Even before we knew atoms existed, we were using them to create new materials and generate energy.
In recent years, scientists have succeeded in creating new atoms: By combining existing nuclei with new superheavy nuclei, they have created 20 elements that do not exist in nature. These man-made atoms disintegrate immediately, but stable variations may not be far behind. After all, it didn't even take us a hundred years to build nuclear power plants and atomic bombs after unlocking the inner energy of the atomic nucleus.
Today, physicists are searching for the even smaller components that make up atoms - quarks, leptons and bosons. New findings in this still mysterious field could even rewrite our understanding of the universe.
Levent Aslan
25 January 2025



Add Comment